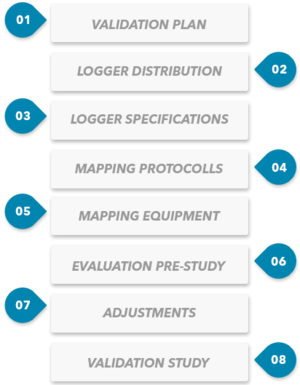
8 steps to GMP compliant Mapping of storage rooms
The requirements of the worldwide GMP (Good Manufacturing Practice) regulations regarding storage and logistics are unanimous in one point. When it comes to monitoring storage rooms and warehouses, it must be proven where the weak points are located regarding temperature distribution.
This step-by-step approach helps you to successfully plan and execute the mapping. It helps you to identify the most relevant aspects of the validation, especially the risk assessment regarding the weak points in the temperature and humidity distribution.
Routine monitoring:
The ideal data loggers for routine monitoring are the data loggers of the EBI-25 and EBI-20 family. Ensure that your monitoring covers all critical points in your warehouse and meets the high standards set for manufacturers and suppliers in the pharmaceutical sector.
GxP compliant mapping
Our Service offer supports you in the planning and implementation of your mapping study.
Stay on top of things with our mapping service:
- Comprehensive preliminary talk
- Provision of calibrated ebro® rental loggers
- Competent evaluation of the measurement results
- Detailed final discussion
Benefit from our expertise - GxP compliant mapping of:
- Production rooms
- Refridgerators and freezers
- GxP-Warehouses
A successful mapping study is not only a prerequisite for operating a GMP warehouse. It is essential for operators if knowledge about the temperature distribution within the warehouse or the cold rooms is to be gained and the performance of the air conditioning is to be assessed.
Do you operate a GMP warehouse and have not yet completed the qualification? Have there been serious changes to your storage area recently, so that you need to carry out requalification?
No problem! Contact our expert Sebastian Schwarz by email or phone +498419547837 or directly your contact person in the region for an appointment for a winter mapping or for a summer mapping.